
Pharmaceutical QC
Raw material verification, contaminant detection, and GMP compliance solutions.
Pharmaceutical quality control demands the highest standards of accuracy and compliance. Our spectroscopy solutions support raw material identification, contamination detection, and process monitoring throughout the pharmaceutical manufacturing lifecycle.
Our Solutions
Raw Material ID
Rapid identity verification of incoming raw materials with 21 CFR Part 11 compliance.
- USP <1120> compliant
- Pass/fail results in 30 seconds
- Electronic signatures
Particulate Analysis
Detection and identification of visible and sub-visible particles in injectables.
- Particles down to 5μm
- USP <788> support
- Root cause analysis
Compliance & Standards
Raman Spectroscopy
Electronic Records; Electronic Signatures
Recommended Products
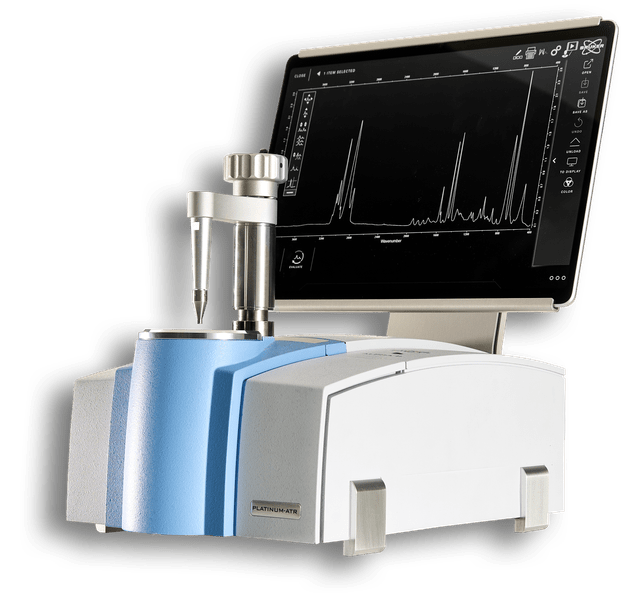
ALPHA II
The Bruker ALPHA II is a compact, robust FT-IR spectrometer designed for high-precision laboratory analysis and routine quality control with an intuitive, user-friendly interface. The Swiss-knife of FT-IR spectroscopy.

LUMOS II FT-IR Microscope
The LUMOS II is a stand-alone, fully automated FT-IR microscope designed to provide exceptional chemical imaging performance and ease of use for both novice and expert researchers.

TANGO II FT-NIR Spectrometer
The Bruker TANGO II is a robust, high-throughput FT-NIR analyzer designed to provide fast, reliable, and "one-button" quality control for liquids, solids, and inhomogeneous materials in industrial environments.

BRAVO Handheld Raman Spectrometer
The BRAVO is a high-performance, handheld Raman analyzer designed for rapid material identification and raw material verification, featuring patented fluorescence mitigation for even the most challenging samples.
Resources & Downloads
Frequently Asked Questions
How does FT-NIR ensure GMP compliance in pharma?
FT-NIR enables 100% ID testing of incoming raw materials in warehouse quarantine, compliant with PIC/S and GMP guidelines. It provides a distinct 'pass/fail' result in seconds without opening containers.
What is the USP <1120> standard?
USP <1120> provides the guidelines for Raman spectroscopy in pharmaceutical applications, covering instrument qualification, method validation, and operational procedures for quality control.
Can Raman microscopy detect foreign particles?
Yes, Raman microscopy is ideal for root cause analysis of particulate contamination in injectables. It can identify the chemical composition of particles down to a few microns to trace their source.
